
What is a Clinical Trial Management System (CTMS)?
Are you struggling to track costs and manage patient data during your clinical trials?
Clinical trial management systems (CTMS) can revolutionize the way you conduct clinical trials, improving efficiency and compliance.
This article explores the basics of CTMS, key features, choices between off-the-shelf and custom solutions, and the significant benefits of implementing a CTMS in your organization.
Discover how platforms like Openkoda can accelerate the development of custom CTMS solutions tailored to your specific needs.
The Basics: What is a CTMS System and Why Is It Important?
A Clinical Trial Management System (CTMS) is a comprehensive software solution designed to manage and streamline the various aspects of conducting clinical trials.
These systems are integral to the planning, tracking, and management of clinical research activities. They provide a centralized platform that facilitates the organization of trial data, monitoring of progress, and ensuring compliance with regulatory requirements.
For instance, a pharmaceutical company can use a CTMS to oversee the entire lifecycle of a clinical trial, from participant recruitment and clinical trial data collection to analysis and reporting.
By automating and integrating these processes, CTMS systems help organizations enhance efficiency, reduce costs, and improve the accuracy and reliability of trial outcomes.
Six Key Features of a CTMS
Building on the basic understanding of what a Clinical Trial Management System (CTMS) is, it’s important to explore the key features that make these systems indispensable to organizations conducting clinical trials.
These features provide the tools necessary to effectively and efficiently manage complex clinical research processes, including:
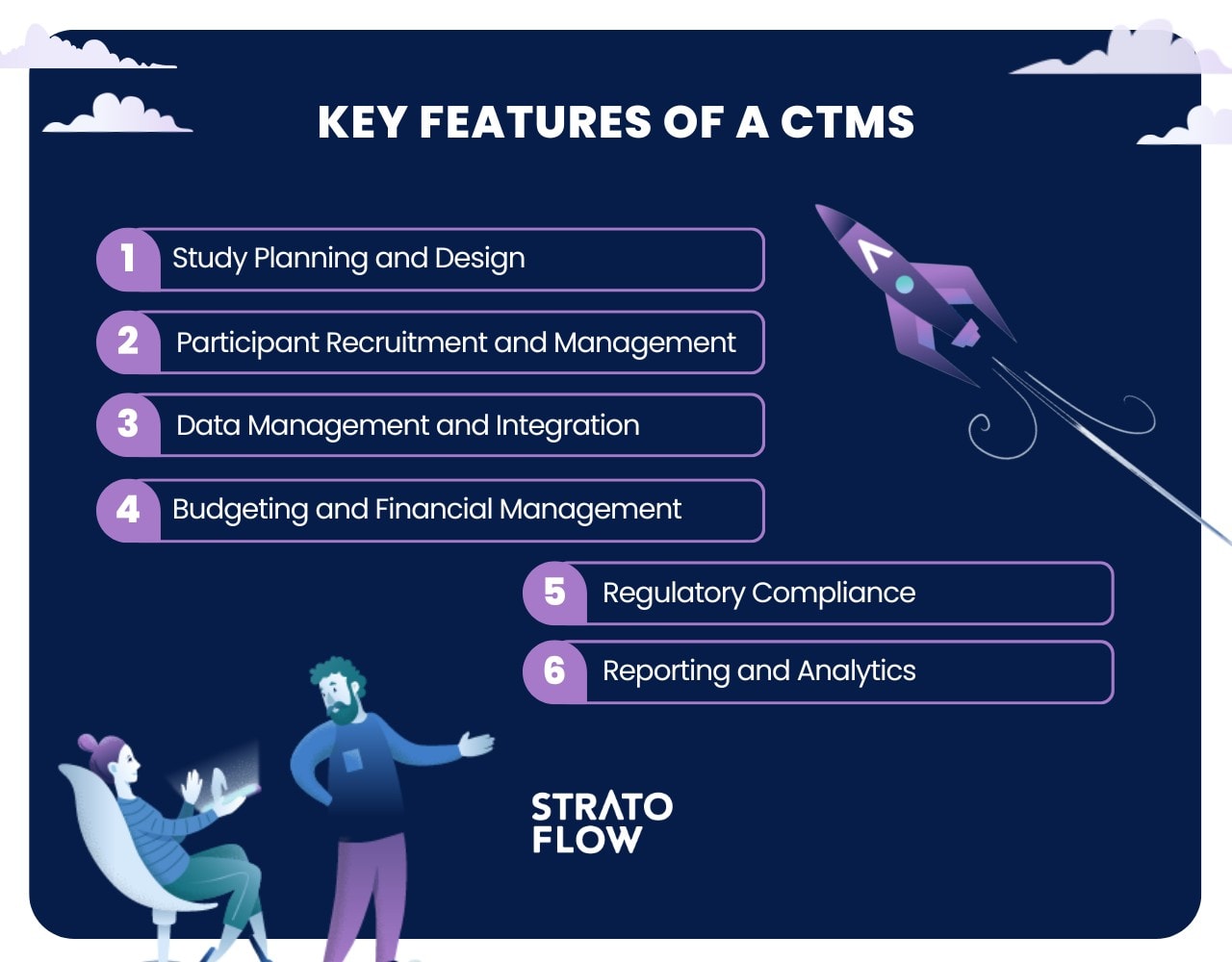
Study Planning and Design
A CTMS enables meticulous planning and design of clinical trials, allowing researchers to outline protocols, set goals and allocate resources efficiently.
This function ensures that every aspect of the study is carefully considered and documented before the study begins.
The process typically involves defining the study design, developing a detailed study plan, establishing timelines, and assigning roles and responsibilities.
Importantly, it also includes coordination with research sites to ensure that all sites are prepared and equipped for the study. This thorough preparation helps anticipate challenges and provides a clear path for conducting the study.
Participant Recruitment and Management
Patient recruitment is a critical and often challenging part of clinical trials.
A CTMS streamlines this process by providing tools to track recruitment status, manage participant information, and monitor compliance with study protocols.
It facilitates communication with participants, schedules visits, and ensures that all necessary consents are obtained and properly documented. By optimizing patient recruitment, CTMS systems help ensure that studies have the necessary enrollment to produce reliable results.
Data Management and Integration
Effective data management is at the heart of any successful clinical trial.
CTMS systems provide robust capabilities for storing, integrating and collecting patient data from multiple sources.
Given the sensitive nature of patient data, CTMS systems prioritize data security, employing advanced encryption and access control mechanisms to protect against unauthorized access and breaches.
This ensures data quality and integrity and facilitates real-time access and analysis, which is critical for making informed decisions throughout the trial.
Regulatory Compliance
Ensuring compliance with regulatory standards is critical in clinical trials.
CTMS systems help organizations maintain compliance by providing functions for managing regulatory documents, tracking approvals, and maintaining audit trails.
These systems must comply with standards and regulations such as Good Clinical Practice (GCP), International Council for Harmonization (ICH) guidelines, and regional regulations such as the FDA in the United States or the EMA in Europe.
This compliance ensures that the trial meets all legal and ethical requirements, protecting both participants and data integrity.
Budgeting and Financial Management
Managing the financial aspects of clinical trials can be complex.
A CTMS includes budgeting, financial and project management aspects that allow organizations to track expenses, manage contracts, and ensure that the trial stays within budget.
Reporting and Analytics
Accurate and timely reporting is critical to the success of clinical trials.
CTMS systems offer comprehensive reporting and analysis tools that provide insight into study progress, participant data, and overall study results. These tools enable better decision making and strategic planning.
Together, these key features improve the efficiency, accuracy, and compliance of clinical trials, making CTMS an invaluable asset for pharmaceutical and medical research organizations.
If your organization needs all of these features, but also requires a customized approach, Openkoda may be an attractive option.
Openkoda is a rapid software development platform with many pre-built components and application templates, making it an excellent fit for developing custom CTMS software.
It allows for flexibility and customization, ensuring that the system can meet the unique needs of your clinical trials.

To explore how Openkoda can transform your clinical trial management, check it out for yourself!
Book your own Openkoda demo today!
Choosing The Right CTMS for Your Company: Ready Made Solution vs. Bespoke Approach
Choosing the right Clinical Trial Management System (CTMS) is a critical decision that can significantly impact the success of your clinical trials.
Companies need to weigh the pros and cons of off-the-shelf solutions versus customized approaches to determine which best meets their specific needs.
How to Choose the Right Clinical Trial Management System
There are several key considerations when selecting a CTMS.
First, evaluate the system’s functionality to ensure that it covers all necessary functions, such as trial design, patient recruitment, data management, regulatory compliance, financial management, and reporting.
It’s also important to evaluate the system’s scalability to accommodate the growth of your clinical trials and its adaptability to integrate with existing technologies and workflows.
Ease of use is also important; the system should be intuitive and easy for your team to learn and use.
In addition, make sure the CTMS offers robust data security measures to protect sensitive patient information and complies with relevant regulatory standards.
Finally, consider the vendor’s reputation and support services, as reliable customer support can be invaluable in resolving issues and ensuring smooth operations.
In such a niche industry, however, finding the perfect solution to meet your organization’s unique needs can be challenging, so a customized approach may be the best way to move forward.
Benefits of Going with a Custom Approach
Choosing a custom CTMS can provide significant benefits tailored to your specific needs. Custom solutions offer greater flexibility, allowing you to tailor the system to your unique processes and workflows.
This customization can lead to increased efficiency and productivity as the software is perfectly aligned with your operational needs.
A customized CTMS can also integrate more seamlessly with other systems and tools your company uses, creating a cohesive technology ecosystem. This integration minimizes disruptions and improves the flow of data across platforms.
Investing in a custom CTMS may involve a higher upfront cost, but the long-term benefits of having a system that fits your needs exactly can outweigh the initial expense.
It provides the opportunity to innovate and implement advanced features specific to your clinical trials, giving you a competitive edge in the research landscape.
But even the cost aspect of a custom clinical trial management system can be somewhat mitigated by the clever use of modern development platforms like Openkoda.
Let’s see how such a tool can increase the development speed and reduce the cost of custom CTMS software solutions.
Openkoda: Speeding Up the Development Speed of Custom Clinical Trial Management Systems
Openkoda is a powerful rapid software development platform designed to accelerate the creation of custom Clinical Trial Management Systems (CTMS).
By leveraging pre-built components and application templates, Openkoda significantly reduces development time and costs, making it an ideal solution for organizations seeking a custom CTMS.
Openkoda’s platform offers a variety of industry-specific templates that serve as a robust foundation for developing custom applications.
These templates include essential features and functionality that can be easily customized to meet specific business needs, allowing companies to quickly build and customize their CTMS without having to start from scratch.
By using Openkoda, companies can achieve up to 60% faster development times compared to traditional methods.
This rapid development capability is particularly beneficial in the niche and highly regulated area of clinical trials, where time and accuracy are critical.
We are currently working on a clinical trial management system template that will include all the key functionalities like:
- Centralized Calendar and Personalized Calendars of Planned Visits: Streamline scheduling with a unified calendar system for all visits.
- Timeline and Milestone Tracking: Stay on track with Gantt charts, calendars, and reminders for key project milestones.
- Resource Management: Efficiently allocate and track resources including staff, equipment, and facilities.
- Budgeting and Financial Management: Create budgets, track expenses, and generate financial reports seamlessly.
- Regulatory Document Management: Store and track all regulatory documents in one secure location.
- Centralized Communication Tools: Facilitate seamless interaction with site personnel through integrated communication tools.
- Patient Database: Manage patient information with a comprehensive and easy-to-use database.
- Patient Communication: Enhance patient engagement with tools for reminders and notifications.
- Electronic Signatures: Simplify the signing process with secure electronic signatures.
- Automatic Document Creation: Generate reports and other documents automatically to save time and reduce errors.
Think of all these features as a foundation upon which you can build your own customized CTMS application without wasting valuable time and resources developing the most basic functionality.
But there’s one more key thing that sets Openkoda apart from the competition – its open source core.
Openkoda’s open architecture ensures that companies retain full ownership of their code, avoiding vendor lock-ins and allowing for greater control over their custom CTMS solutions.
This means that any application you build on top of it is yours to do with as you please. No additional fees and no proprietary technology.
Sound interesting?
To see Openkoda’s capabilities in action and understand how it can revolutionize your clinical trial management, book a free demo today!
Benefits of Clinical Trial Management Systems
The use of a Clinical Trial Management System (CTMS) offers numerous benefits that can significantly improve the efficiency and success of clinical trials.
Unlike traditional methods, a CTMS provides a more sophisticated, centralized and integrated approach. Here are some of the key benefits of using a CTMS:
Improved Data Management
One of the most important benefits of a CTMS is its ability to efficiently manage large amounts of data.
Unlike Excel spreadsheets, which can become unwieldy and error-prone, a CTMS provides a structured environment for data entry, storage and retrieval.
This ensures data integrity and enables real-time data access and analysis, which is critical for making informed decisions throughout the trial process.
Enhanced Compliance and Regulatory Adherence
Compliance with regulatory standards is a critical aspect of clinical trials.
A CTMS helps ensure that all trial activities comply with regulations such as Good Clinical Practice (GCP) and guidelines set by bodies such as the FDA or EMA.
The system automates the tracking and management of regulatory documents, audit trails and approvals, reducing the risk of non-compliance and potential penalties.
Streamlined Project Management
Managing a clinical trial involves coordinating various tasks, schedules, and resources.
A CTMS provides robust project management tools that enable seamless planning, monitoring, and execution of trial activities.
It allows for effective resource allocation, timeline management, and task tracking, ensuring that the trial progresses smoothly and stays on schedule.
Better Patient Recruitment and Retention
Effective patient recruitment and retention are critical to the success of clinical trials.
A CTMS streamlines the recruitment process by managing participant information, tracking recruitment status, and facilitating communication with participants.
This systematized approach improves recruitment efficiency and increases participant retention by ensuring consistent follow-up and engagement.
Cost Efficiency
While the initial investment in a custom software development project may seem substantial, the long-term cost savings are significant.
A CTMS reduces the manual effort required to manage data, ensures compliance, and improves overall trial efficiency.
Compared to the labor-intensive process of managing trials with Excel spreadsheets, a CTMS minimizes errors, reduces redundant tasks, and accelerates the trial timeline, ultimately leading to cost savings.
Final Thoughts
In conclusion, adopting a CTMS can transform the way clinical trials are conducted, offering improved data management, enhanced compliance, streamlined project management, better patient recruitment, and cost efficiency.
These benefits underscore the value of CTMS for companies looking to optimize their clinical trial processes.
Related Posts
Thank you for taking the time to read our blog post!

